
Diamond
Element Case Studies
Nitrogen
From a chemistry standpoint, one of the most daunting ďacid testsĒ imaginable for the new model would be to explain the bizarre properties of Nitrogen - possibly the most enigmatic of all elements. In its diatomic gaseous form it is so inert that Lavoisier was compelled to label it Azote, (meaning without life) yet itís myriad of compounds are extremely volatile as a main component of explosives, and many other highly reactive compounds. It also exhibits a profusion of 8 valence states from -3 to +5. No other element comes even close. Obviously, there is something extremely peculiar about the atomic structure of Nitrogen that must logically explain such very unique properties.
What is behind all this strange behavior is actually quite easily explained by its cubic close packed nuclear geometry as illustrated in figure 1 in the left margin. Nitrogen's Base 2 Octahedral neutron core (B2 Octacore) is identical to that of diamond (figure 2) composed of six neutrons with 7 nested protons (hydrenos) and one additional peripheral neutron which provides perfect cubic symmetry of the nucleus as clearly evident in figure 3. The energy density and frequency distribution of the local vacuum has evidently been attenuated and taxed to its limit, barring the formation of the eighth hydreno which consequently remains in its unexcited state as a peripheral neutron (figure 4).
This implies that the valence states are poorly defined, subject to wide variations (accommodated all 8 valence configurations) and potentially very unstable. All eight nested bond sites on the neutron core are absolutely identical which logically infers that any of the 7 hydreno positions is apt to exchange states with the one peripheral neutron with very little aggravation, especially in explosive compounds such as TNT or Nitro-Glycerin, causing extremely volatile reactions involving the total collapse of any one of the seven hydrenos.
The entire quantum energy of the affected orbital is consequently liberated, releasing a massive burst of thermal radiations, while the neutron simultaneously expands to form a new hydreno to replace the one that is collapsing, gathering its required sustaining energy from the discrete harmonic frequencies of the local vacuum flux. This complete collapse of a valence orbital yields the maximum energy possible in a chemical reaction, if it is even appropriate to refer to it as such. Given that the nitrogen atom has in effect changed its nuclear structure, this is more properly referred to as a form of hyperchemisty or chemo-nuclear reaction. During the reaction transient there is evidently 8 hydrenos (protons), 2 of which are exchanging states, and only 6 core neutrons (the identical composition of unstable Oxygen 14).
In contrast diatomic nitrogen creates a virtually impenetrable chemical barrier composed of radially symmetric distribution of 14 hydrenos of essentially identical quantum energy states, none of which are free to react. The two atoms are firmly interlocked at the bond interface defined by the collapsed orbital of the two peripheral neutrons, preventing them from assuming an excited hydreno state.
Furthermore, the cause of the hexagonal close packed crystal structure of solid nitrogen is readily apparent from the six staggered facets formed by the hydreno grouping defined from the neutron core extrapolations. The cryogenic fluid nature of nitrogen is also revealed by the lack of significant charge polarity of the diatomic atom. Water by comparison forms a fluid at much higher temperatures due to its strong polarity and dielectric properties.
This closely bound diatomic gas is also known to undergo endogenous transformation from thermally excited N2 to form carbon monoxide with the exchange of a proton-neutron pair (deuteron) between the two closely bound nuclei. This discovery by Dr. Louis Kervran lead to major changes in welding practices that had caused numerous deaths due to CO poisoning that could not be traced to the work environment.
In contrast to Lattice Nested Hydreno model, the Bohr-Rutherford planetary model is irremediably inconsistent with such logical determinism and ought to be largely abandoned as plainly inferior theory of matter.
For a more thorough comprehension please purchase the ebook and help pay my mortgage. Stay Tuned as we add more Case Studies.....
© 2006, 2007

Neutron Octacores

CCP Nuclear Lattice
Base 2
Base 5
.jpg)
.jpg)
.jpg)
Nitrogen Figure 1
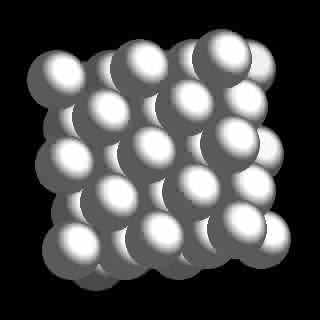
Cubic Close Pack Geometry
Figure 2
Figure 3
pn
Figure 4
Seven Hydreno View
(Updated Mar 05/07)