Double click to edit

Aluminum Nucleus
B2 Cuboctacore
B2 Cuboctacore
Element Case Studies
Without going into a lot of detail, these short case studies are intended to demonstrate the extraordinary predictive value and deterministic logic of the Lattice Nested Hydreno Model as it applies to a wide array of atomic and nuclear properties and related phenomena. For a fuller comprehension of the underlying concepts described, please purchase the ebook.
Aluminum
Aluminum is a rather unique metal in many respects, possessing a high strength to weight ratio; a single valence state of +3; one stable isotope and cubic close packed (ccp) crystal structure. The question is, can these and many other properties of Aluminum be logically accounted for by the new atomic model? The answer is a resounding yes.
First of all why does aluminum possess only one stable isotope? This occurs at quite regular intervals throughout the periodic table, but the fundamental cause is not known. Conventional Bohr-Rutherford atomic theory and its refinements provides no means of comprehension, even though the answer is evidently quite simple, as clearly predicted by the Hydreno atomic model. The solution to this riddle turns out to be purely a matter of the permissible geometries and symmetry of the nucleus.
Consulting the table of nuclear geometries (provided in the ebook) one quickly finds a combination of core neutrons and surface nested bond sites that would appear to satisfy the requirement for 27 nucleons with a core of 13 neutrons, 13 surface nested protons (hydrenos) and one vacant site for an additional peripheral neutron. This combination produces a perfect, and complete geometric shape that cannot accommodate any more nucleons without disrupting the minimum required symmetry of the nucleus restricting the element to a single stable isotope.
Furthermore the resulting charge asymmetry of the nucleus produced by the lone peripheral neutron produces the requisite nuclear and atomic polarity required for self alignment of the elemental crystal solid exhibiting the ccp crystal lattice providing the observed 12 points of contact with adjacent atoms. This crystal arrangement quite obviously results from a simple extrapolation of the hydreno sites to produce 12 clearly defined facets of the base 2, cuboctahedral neutron core (B2 Cuboctacore) lattice, which also contributes to aluminums strength, particularly in alloyed form.
Looking down on the one peripheral neutron, (figure 2) the 3 high-energy hydreno, valence positions become immediately evident. These are the hydrenos in the most physically pronounced positions with greatest exposure to the sustaining frequencies of the quantum vacuum flux. These protons are clearly preferred due to their proximity to the peripheral neutron since it does not have a quantum orbital state to attenuate and shadow the local vacuum flux, leaving more energy to sustain these three valence positions. Indeed, the peripheral neutrons are a major contributing factor to the observed valence states for all the elements. In profusion, they also give rise to the many stable isotopes of elements such as Tin, which has a full slate of ten.
A myriad of other properties can also be explained in a similar logical and frequently intuitive fashion. In contrast to the Lattice Nested Hydreno model, the Bohr-Rutherford planetary concept is, irremediably inconsistent with such logical determinism and ought therefore to be largely abandoned as a plainly inferior model of physical reality.
Stay Tuned as we gradually add more Case Studies.....
© 2006, 2007
.jpg)
Neutron Cuboctacores
base 2
base 4


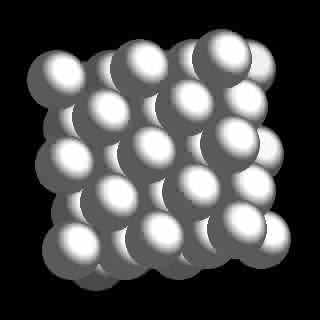
Cubic Close Packed
Atomic Crystal
#1
Peripheral Neutron View
#2
#3
Figure 2
Another Perspective
pn
(Updated Mar 5/07)