
Germanium
.jpg)

.jpg)
Rhodium Nucleus
Arsenic
Cadmium 116
A Critical Review Of The Atomic Theories
Of Bohr and Rutherford
(Excerpts From The Book)
Objections To The Bohr-Rutherford Model
Having repeated the heretical assertion that the neutron is just a bound state of hydrogen, or more simply the sum of a proton and electron, it becomes necessary to take a closer look at other aspects of existing atomic theory with particular emphasis on the still widely accepted Bohr-Rutherford planetary model. Aside from its undisputed accuracy in the prediction of the spectral lines of hydrogen, the Bohr-Rutherford atomic model appears to be in need of some serious rework. The most glaring objections come from chemistry since Bohr’s model is unable to logically account for chemical bonding, isomers, allotropic forms and many other properties resulting in an artificial rift between nuclear physics and chemistry, which remains tied to a more definitive structure of the atom as advocated by Valence theorists of the likes of Thompson, Parson, Lewis and Allen. All of whom raised valid objections to the Bohr model from the outset....
These largely ignored objections where succinctly enumerated by A. Crehore[28] who rightly observed that the “entire field of chemistry is not a silly thing to be light heartedly dismissed in order to embrace the Bohr atom”. Furthermore, both Crehore and Allen pointed out that the useful ideas from Bohr’s theory such as the explanation of series lines in spectra can be obtained from other models, that do not ignore for convenience, basic laws of electromagnetism, concerning the behavior and interaction of charged particles. Bohr’s insistence that the accelerated motion of electrons within the atom do not radiate is logically objectionable. The complex EM interactions between the hundreds of “particle” electrons presumed to be orbiting large nuclei in a very complex fashion would be hopelessly intricate and more aptly described as a state of indeterminate chaos....
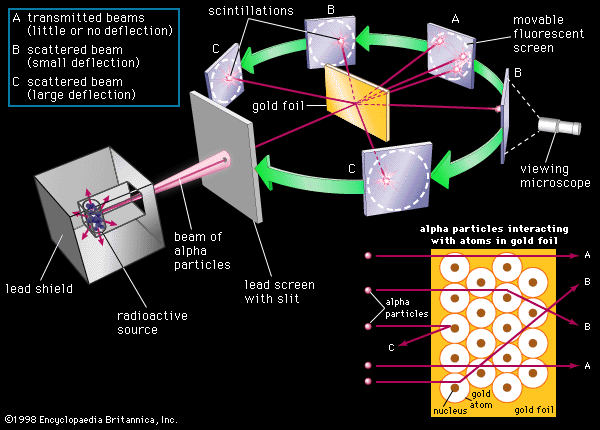
Another objection is raised regarding Rutherford’s[29] calculation of the size of the nucleus, which could be off by a very wide margin, due to several invalid assumptions including the erroneous exchange of terminology used in his analysis that permitted the “surface” of the nucleus, to be considered equivalent to the “center” of the nucleus. From the outset, Rutherford presumes a point like nature for the nucleus with a dimension conjectured to be less than 10-14 m. If the nuclear charge is in reality dispersed over a considerably larger volume than the presently conjectured 10-42 m3, the coulomb interaction with a high velocity charged particle would be much different than Rutherford’s presumption. A larger nucleus, with a much reduced electric field intensity, would permit the high energy alpha particles used in the Gold foil experiment to come much closer to the nucleus, possibly striking it.
Rutherford also makes the mistake of presuming that the probability of large angle deflections is directly related to the thickness of the gold foil, ignoring the obvious tendency of the alpha particle to be channeled within the highly organized atomic lattice by the presence of the concentrated charge of the gold nuclei, and secondarily by the electron cloud surrounding it. The beam trajectory being normal with respect to the foil, and therefore parallel to the internal lattice structure, would effectively prevent large angle deflections beyond the first two staggered layers of the cubic close-packed crystal structure. Barring a direct hit, or very close miss on a gold nucleus within these first two layers, the high energy alpha particle would simply continue on its merry way between the nuclei, effectively channeled through the entire foil, without any further prospects of high angle deflections. Only small deflections would be possible for the remainder of its travel, and these would be entirely insufficient to bump the alpha particle out of the defined channel provided by the lattice....
The thickness of the foil used in the experiment is cited, near enough, as 10-8 m. Given the well-established atomic dimension of 10-10 m, the foil was less than 100 atoms thick, making it extremely unlikely that a single alpha particle could experience more than one large angle deflection. The proportion of large angle deflections greater than 90o was found to be only one in 20,000. Following the reasoning above, about half of these deflections happen at the surface of the foil, the remainder occurring at the staggered layer of atoms immediately below the surface of the foil of the ccp structure. In other words if the foil could have been reduced to only two layers thick the number of high angle deflections would have been essentially the same as that observed for the 100 atom thick foil. The remaining 98 atomic layers would have virtually no effect with regard to high angle scattering. Rutherford’s inclusion of a thickness factor in his probability analysis would therefore appear quite unjustified, and would by itself give rise to a 100 fold error in the size of the nucleus....
Making no presumptions about the relative size of the nucleus, and the nature of the Coulomb interaction, a reasonable estimate could be asserted by considering only the high angle scattering at the surface of the foil, which clearly indicates that only about 1/10,000 of the surface area is occupied by something substantial (a heavy nucleus); the remainder being mostly empty space occupied by the orbital electron clouds. This clearly and simply implies that the diameter of the nucleus could conceivably be roughly 1/100 of the atoms diameter. The result of this “ball park” analysis is that the nuclear radius may be about a thousand times larger than the presently conceded dimension of 10-15 m, which results in an atomic diameter in the range of 10-10 m, with a nucleus of 10-12 m; a much different view from that presently accepted. Even at 10-12 the absolute Casimir pressure is still a very substantial 1018 kPa and certainly adequate to hold the nucleus together against the substantially reduced coulomb repulsion of the protons....
A thousand fold increase in the nuclear diameter, also results in a million fold reduction in the intensity of the mutual coulomb repulsion of the protons of the rarified nucleus, assuming for simplicity a radial field attenuation, according to the normal 1/r2 relation. Such a dramatic reduction greatly improves the prospects of fusion at low energies and calls into question the present understanding of thermonuclear explosions, which are heavily reliant on the notion of an extremely intense coulomb repulsion within the nucleus....
Thermal neutron absorption cross-sections cast further doubt on the notion of an extremely small nucleus. Gold 198 and Cadmium 113 for instance have cross-sections approaching 27,000 barns, which on a statistical basis, just so happens to equate to an apparent nuclear dimension of 10-12 m, again a thousand times larger than the presently accepted nuclear dimension. Furthermore Gd157 tops the list for thermal neutron capture with a whopping cross-section of 240,000 barns, yielding an apparent nuclear dimension approaching 10-11m. By comparison the readily fissionable material, U235 has a combined thermal neutron cross-section of only 700 Barns. Obviously there are some very peculiar characteristics of the nucleus that on the one hand give rise to such incredible high cross-sections while some metals, such as Zirconium, appear to be essentially transparent to neutrons with cross-sections measured in milibarns. It should be apparent from the reality of the ZPF and ultra-close range Casimir effects it is quite evident that these wide variations in nuclear cross-section might be straight forwardly explained....
Neutron radiography provides even more doubts and questions about the minute size of the nucleus. How is it that such sharp, high contrast images can be produced when the neutron beam is only supposedly interacting with an extremely small, essentially non-existent nucleus? One would expect the transmission losses through the target material, resulting from reflection and absorption, to be too small to produce sharp images on film. All of this discussion has for the moment discounted the very real prospect of an incident neutron being converted to a hydrino or hydrogen atom, as the surface bound electron jumps back to some higher quantum state, in response to the forces and energy of interactions in the target material. This seems especially likely in respect to hydrogenous materials, which despite their small nuclei, are known to provide very effective shielding for neutrons....
The presence of a high concentration of hydrogen atoms would presumably organize the local vacuum flux to provide a strong background signal for the production of hydrogen from neutrons, which just so happens to be the normal decay route for neutrons. Along this line of reasoning it should again be emphasized that matter always organizes the energy of the vacuum in much the same way that diffused white light from the sun is organized by bulk matter to create complex images of form, color and texture. Organization of the quantum vacuum energy is however primarily accomplished by the sub-atomic and sub-nuclear structure of matter apparently expressing itself as the inertial and gravitational properties of the atomic assembly as well as other phenomena....
Without a doubt, there is lengthy list of anomalies, not too mention familiar phenomena, that cannot be adequately explained by existing atomic theory including, for instance: atomic and nuclear bonding, isotopic distributions (or lack thereof), missing elements (Tc, Pm), crystal structure, bond angles, allotropes, isomers and so on ad nauseam. Why is it that no stable isotopes exist for elements having an odd number of neutrons and protons? Why is it that only one stable form exists for over 25 elements while others have up to 10? What is behind the enigmatic chemistry of Nitrogen. Why does the hot fission of Uranium result in unequal fission fragments. How does cold fission frequently occur without radioactivity or the release of the vast amounts of energy characteristic of thermonuclear reactions. Why is it that no one seems to even bother anymore with such fundamental questions?
Taken collectively, these numerous objections to the Bohr - Rutherford model clearly indicate that conventional atomic theory is way out of whack with reality and needs a major upgrade, if not a total rebuild from the ground up. A good place to start would be a fresh look at some of the history of the atom, which has been thoroughly researched and succinctly reported recently by R. Monti. A high point of this riveting, historical synopsis is the Alpha Extended model originally proposed by W. Harkin [30]. Resurrected and embellished by Monti, the Alpha extended model provides some interesting theoretical framework for a more complete understanding of low energy induced fusion and fission reactions, both of which have been clearly demonstrated to occur with surprising abundance in a wide variety of low energy systems....
Having now cast a long shadow of doubt on existing notions of the atom, it is high time to begin rebuilding a revised model that is much closer to the observed reality. Adopting a variant of the Mills Hydrino concept, (relabeled the Hydreno for distinction) combined with further refinements of the Alpha Extended model of Harkin and Monti, and the recent discovery of the “impossible” tetraneutron, yields a simple, yet very revolutionary concept....
© 2006
Of Bohr and Rutherford
(Excerpts From The Book)
Objections To The Bohr-Rutherford Model
Having repeated the heretical assertion that the neutron is just a bound state of hydrogen, or more simply the sum of a proton and electron, it becomes necessary to take a closer look at other aspects of existing atomic theory with particular emphasis on the still widely accepted Bohr-Rutherford planetary model. Aside from its undisputed accuracy in the prediction of the spectral lines of hydrogen, the Bohr-Rutherford atomic model appears to be in need of some serious rework. The most glaring objections come from chemistry since Bohr’s model is unable to logically account for chemical bonding, isomers, allotropic forms and many other properties resulting in an artificial rift between nuclear physics and chemistry, which remains tied to a more definitive structure of the atom as advocated by Valence theorists of the likes of Thompson, Parson, Lewis and Allen. All of whom raised valid objections to the Bohr model from the outset....
These largely ignored objections where succinctly enumerated by A. Crehore[28] who rightly observed that the “entire field of chemistry is not a silly thing to be light heartedly dismissed in order to embrace the Bohr atom”. Furthermore, both Crehore and Allen pointed out that the useful ideas from Bohr’s theory such as the explanation of series lines in spectra can be obtained from other models, that do not ignore for convenience, basic laws of electromagnetism, concerning the behavior and interaction of charged particles. Bohr’s insistence that the accelerated motion of electrons within the atom do not radiate is logically objectionable. The complex EM interactions between the hundreds of “particle” electrons presumed to be orbiting large nuclei in a very complex fashion would be hopelessly intricate and more aptly described as a state of indeterminate chaos....
Another objection is raised regarding Rutherford’s[29] calculation of the size of the nucleus, which could be off by a very wide margin, due to several invalid assumptions including the erroneous exchange of terminology used in his analysis that permitted the “surface” of the nucleus, to be considered equivalent to the “center” of the nucleus. From the outset, Rutherford presumes a point like nature for the nucleus with a dimension conjectured to be less than 10-14 m. If the nuclear charge is in reality dispersed over a considerably larger volume than the presently conjectured 10-42 m3, the coulomb interaction with a high velocity charged particle would be much different than Rutherford’s presumption. A larger nucleus, with a much reduced electric field intensity, would permit the high energy alpha particles used in the Gold foil experiment to come much closer to the nucleus, possibly striking it.
Rutherford also makes the mistake of presuming that the probability of large angle deflections is directly related to the thickness of the gold foil, ignoring the obvious tendency of the alpha particle to be channeled within the highly organized atomic lattice by the presence of the concentrated charge of the gold nuclei, and secondarily by the electron cloud surrounding it. The beam trajectory being normal with respect to the foil, and therefore parallel to the internal lattice structure, would effectively prevent large angle deflections beyond the first two staggered layers of the cubic close-packed crystal structure. Barring a direct hit, or very close miss on a gold nucleus within these first two layers, the high energy alpha particle would simply continue on its merry way between the nuclei, effectively channeled through the entire foil, without any further prospects of high angle deflections. Only small deflections would be possible for the remainder of its travel, and these would be entirely insufficient to bump the alpha particle out of the defined channel provided by the lattice....
The thickness of the foil used in the experiment is cited, near enough, as 10-8 m. Given the well-established atomic dimension of 10-10 m, the foil was less than 100 atoms thick, making it extremely unlikely that a single alpha particle could experience more than one large angle deflection. The proportion of large angle deflections greater than 90o was found to be only one in 20,000. Following the reasoning above, about half of these deflections happen at the surface of the foil, the remainder occurring at the staggered layer of atoms immediately below the surface of the foil of the ccp structure. In other words if the foil could have been reduced to only two layers thick the number of high angle deflections would have been essentially the same as that observed for the 100 atom thick foil. The remaining 98 atomic layers would have virtually no effect with regard to high angle scattering. Rutherford’s inclusion of a thickness factor in his probability analysis would therefore appear quite unjustified, and would by itself give rise to a 100 fold error in the size of the nucleus....
Making no presumptions about the relative size of the nucleus, and the nature of the Coulomb interaction, a reasonable estimate could be asserted by considering only the high angle scattering at the surface of the foil, which clearly indicates that only about 1/10,000 of the surface area is occupied by something substantial (a heavy nucleus); the remainder being mostly empty space occupied by the orbital electron clouds. This clearly and simply implies that the diameter of the nucleus could conceivably be roughly 1/100 of the atoms diameter. The result of this “ball park” analysis is that the nuclear radius may be about a thousand times larger than the presently conceded dimension of 10-15 m, which results in an atomic diameter in the range of 10-10 m, with a nucleus of 10-12 m; a much different view from that presently accepted. Even at 10-12 the absolute Casimir pressure is still a very substantial 1018 kPa and certainly adequate to hold the nucleus together against the substantially reduced coulomb repulsion of the protons....
A thousand fold increase in the nuclear diameter, also results in a million fold reduction in the intensity of the mutual coulomb repulsion of the protons of the rarified nucleus, assuming for simplicity a radial field attenuation, according to the normal 1/r2 relation. Such a dramatic reduction greatly improves the prospects of fusion at low energies and calls into question the present understanding of thermonuclear explosions, which are heavily reliant on the notion of an extremely intense coulomb repulsion within the nucleus....
Thermal neutron absorption cross-sections cast further doubt on the notion of an extremely small nucleus. Gold 198 and Cadmium 113 for instance have cross-sections approaching 27,000 barns, which on a statistical basis, just so happens to equate to an apparent nuclear dimension of 10-12 m, again a thousand times larger than the presently accepted nuclear dimension. Furthermore Gd157 tops the list for thermal neutron capture with a whopping cross-section of 240,000 barns, yielding an apparent nuclear dimension approaching 10-11m. By comparison the readily fissionable material, U235 has a combined thermal neutron cross-section of only 700 Barns. Obviously there are some very peculiar characteristics of the nucleus that on the one hand give rise to such incredible high cross-sections while some metals, such as Zirconium, appear to be essentially transparent to neutrons with cross-sections measured in milibarns. It should be apparent from the reality of the ZPF and ultra-close range Casimir effects it is quite evident that these wide variations in nuclear cross-section might be straight forwardly explained....
Neutron radiography provides even more doubts and questions about the minute size of the nucleus. How is it that such sharp, high contrast images can be produced when the neutron beam is only supposedly interacting with an extremely small, essentially non-existent nucleus? One would expect the transmission losses through the target material, resulting from reflection and absorption, to be too small to produce sharp images on film. All of this discussion has for the moment discounted the very real prospect of an incident neutron being converted to a hydrino or hydrogen atom, as the surface bound electron jumps back to some higher quantum state, in response to the forces and energy of interactions in the target material. This seems especially likely in respect to hydrogenous materials, which despite their small nuclei, are known to provide very effective shielding for neutrons....
The presence of a high concentration of hydrogen atoms would presumably organize the local vacuum flux to provide a strong background signal for the production of hydrogen from neutrons, which just so happens to be the normal decay route for neutrons. Along this line of reasoning it should again be emphasized that matter always organizes the energy of the vacuum in much the same way that diffused white light from the sun is organized by bulk matter to create complex images of form, color and texture. Organization of the quantum vacuum energy is however primarily accomplished by the sub-atomic and sub-nuclear structure of matter apparently expressing itself as the inertial and gravitational properties of the atomic assembly as well as other phenomena....
Without a doubt, there is lengthy list of anomalies, not too mention familiar phenomena, that cannot be adequately explained by existing atomic theory including, for instance: atomic and nuclear bonding, isotopic distributions (or lack thereof), missing elements (Tc, Pm), crystal structure, bond angles, allotropes, isomers and so on ad nauseam. Why is it that no stable isotopes exist for elements having an odd number of neutrons and protons? Why is it that only one stable form exists for over 25 elements while others have up to 10? What is behind the enigmatic chemistry of Nitrogen. Why does the hot fission of Uranium result in unequal fission fragments. How does cold fission frequently occur without radioactivity or the release of the vast amounts of energy characteristic of thermonuclear reactions. Why is it that no one seems to even bother anymore with such fundamental questions?
Taken collectively, these numerous objections to the Bohr - Rutherford model clearly indicate that conventional atomic theory is way out of whack with reality and needs a major upgrade, if not a total rebuild from the ground up. A good place to start would be a fresh look at some of the history of the atom, which has been thoroughly researched and succinctly reported recently by R. Monti. A high point of this riveting, historical synopsis is the Alpha Extended model originally proposed by W. Harkin [30]. Resurrected and embellished by Monti, the Alpha extended model provides some interesting theoretical framework for a more complete understanding of low energy induced fusion and fission reactions, both of which have been clearly demonstrated to occur with surprising abundance in a wide variety of low energy systems....
Having now cast a long shadow of doubt on existing notions of the atom, it is high time to begin rebuilding a revised model that is much closer to the observed reality. Adopting a variant of the Mills Hydrino concept, (relabeled the Hydreno for distinction) combined with further refinements of the Alpha Extended model of Harkin and Monti, and the recent discovery of the “impossible” tetraneutron, yields a simple, yet very revolutionary concept....
© 2006

Rutherford's Gold Foil Experiment
(Updated Mar 5/07)
Aluminum 27 Nucleus